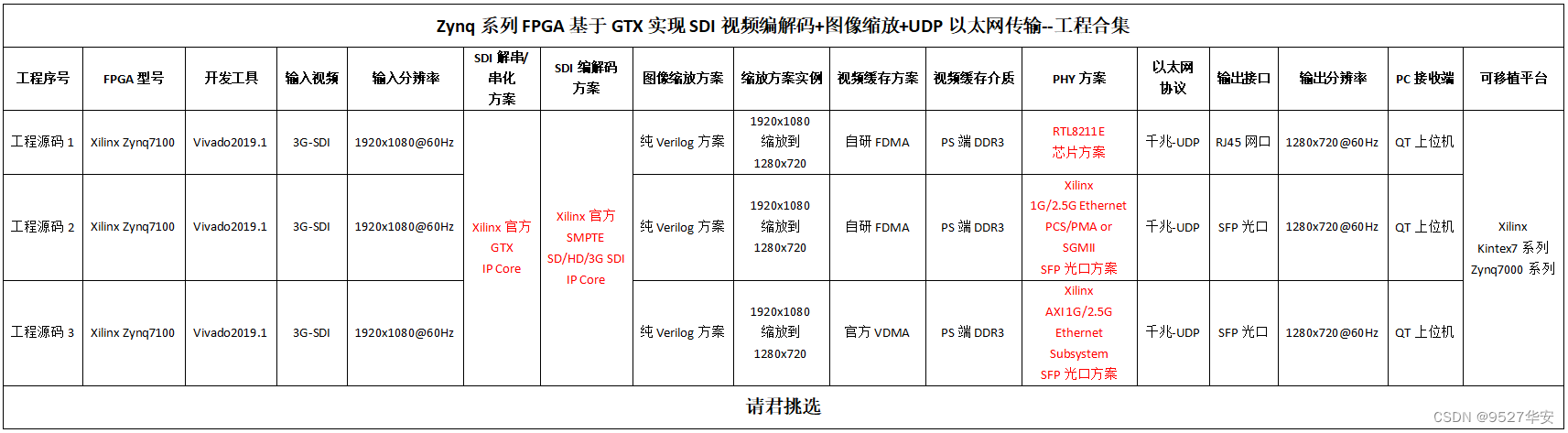
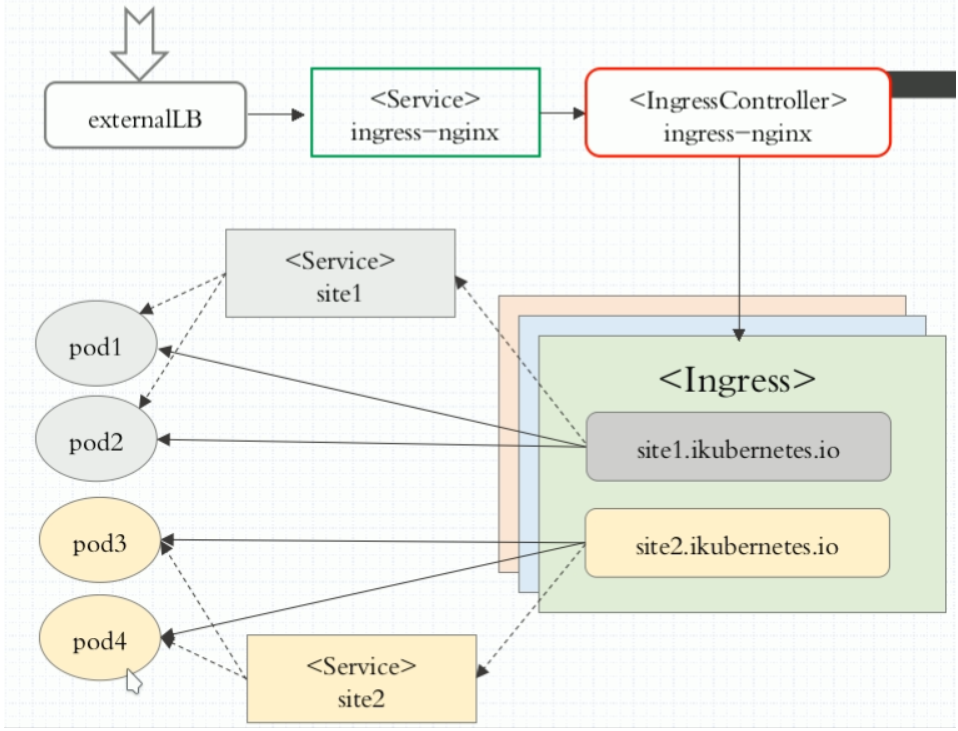
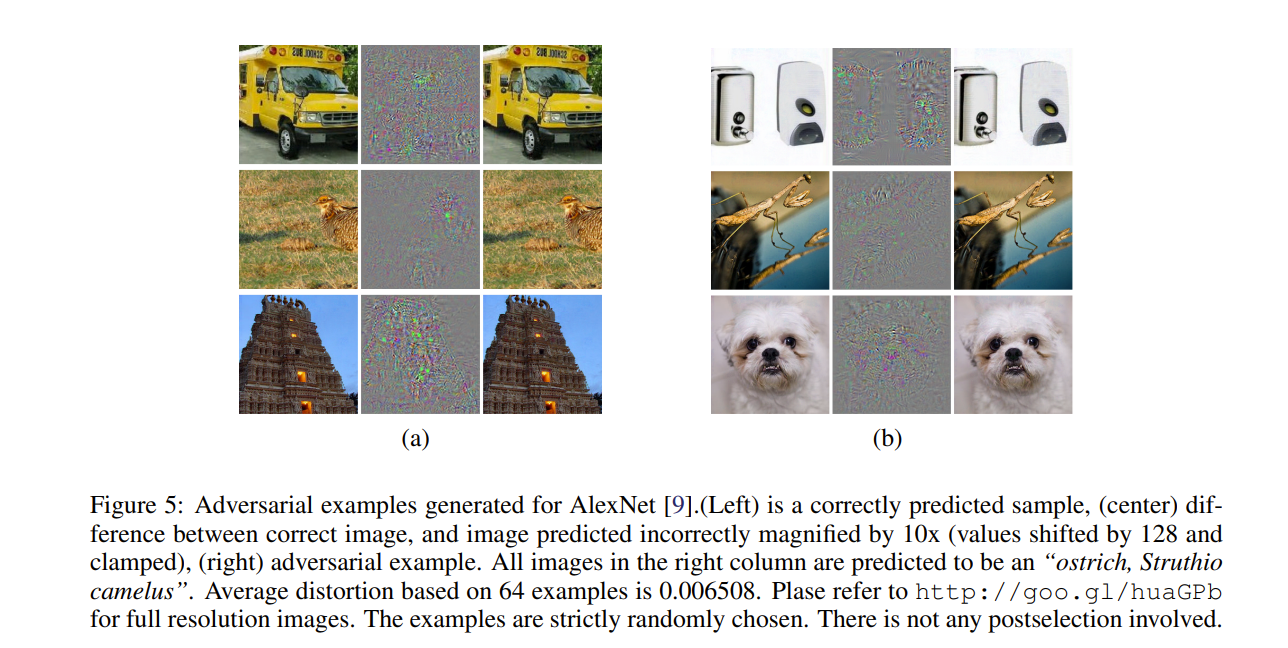
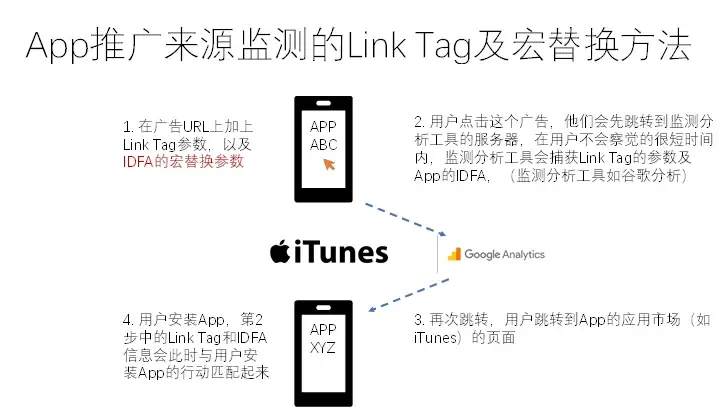
“Publication Figure 6”
本站蜘蛛链接: https://pan.baidu.com/s/15g7caZp354zIWktpnWzWhQ
提取码: 4sh7
Libraries
Standard Import
library(tidyverse)
library(cowplot)
library(scales)
library(ggpubr)
Special
# devtools::install_github("pmartinezarbizu/pairwiseAdonis/pairwiseAdonis")
# library(rgr)
# library(mixOmics)
library(pairwiseAdonis)
library(grid)
library(factoextra)
library(FactoMineR)
Paths
bin_dir = '../bin/'
data_dir = '../data/'
results_dir = '../results/'
Custom Scripts / Theme
source(paste0(bin_dir, 'theme_settings.R'))
source (paste0(bin_dir, 'utilities.R'))
source(paste0(bin_dir, 'species_competition_functions.R'))
source(paste0(bin_dir, 'distmat_utils.R'))
source(paste0(bin_dir, 'analysis_metadata.R'))
date <- format(Sys.time(), "%d%m%y")
Import Tables
Metadata
samples.metadata <- read_tsv(paste0(data_dir, 'samples.metadata.tsv'))
microbe.taxonomy <- read_tsv(paste0(data_dir, 'microbe.taxonomy.tsv'))
microbe.metadata <- microbe.taxonomy %>%
right_join(., read_tsv(paste0(data_dir, 'microbe.metadata.tsv'))) %>%
mutate(gram.bool = ifelse(gram_stain == 'positive', T,
ifelse(gram_stain == 'negative', F, NA)),
spores.bool = ifelse(spore_forming == 'spore-forming', T,
ifelse(spore_forming == 'non-spore-forming', F, NA)))
Taxonomy
MetaPhlAn2 tables
combine with taxonomy
mp_species.long <- microbe.taxonomy %>%
right_join(., read_tsv(paste0(data_dir, 'samples.mp_profiles.species.tsv')),
by = 'species') %>%
left_join(., samples.metadata, by = 'Name')
rCDI subset
annotate with metadata
mp_species.rcdi.long <-
mp_species.long %>%
filter(Study %in% c(rcdi.studies))
Case Summary
SameStr Case-Summary table
sstr_cases <- read_tsv(paste0(data_dir, 'samples.case_summary.tsv')) %>%
left_join(., microbe.taxonomy, by = 'species')
Set Figure
fig = paste0('Fig_6.')
Failed/Resolved Symptoms
Focussing on the difference between successful FMT cases and cases in which symptoms were reported to no have been resolved after FMT treatment.
%Recipient/Donor-derived
Format Data
sstr_cases.rcdi.metrics <-
sstr_cases %>%
filter(Study %in% c('ALM', 'FRICKE')) %>%
filter(Case_Name %in% cases.full) %>%
tag_species_competition(.) %>%
mutate(n = 1) %>%
mutate(source = ifelse(grepl(species, pattern = 'unclassified'), 'Unclassified', source)) %>%
mutate(source = case_when(
analysis_level == 'species' & source == 'self' ~ 'Self Sp.',
analysis_level == 'species' & source == 'donor' ~ 'Donor Sp.',
analysis_level == 'species' & source == 'unique' ~ 'Unique Sp.',
T ~ source
)) %>%
group_by(Study_Type, Case_Name, source, Days_Since_FMT.post, fmt_success.label) %>%
summarize(n = sum(n, na.rm = T),
rel_abund = sum(rel_abund.post, na.rm = T) / 100) %>%
group_by(Case_Name, Days_Since_FMT.post) %>%
mutate(f = n / sum(n, na.rm = T)) %>%
ungroup() %>%
group_by(Case_Name) %>%
mutate(source = case_when(source == 'Unclassified' ~ 'Unclassified Sp.',
source == 'same_species' ~ 'Same Sp.',
source == 'unique' ~ 'Unique to Time Point',
source == 'self' ~ 'Recipient / Initial Sample',
source == 'donor' ~ 'Donor',
source == 'both' ~ 'Coexistence',
T ~ source)) %>%
pivot_wider(names_from = 'source',
values_from = c('rel_abund','f', 'n'), names_sep = '___') %>%
mutate_at(.vars = vars(contains('___')),
.funs = funs(replace_na(., 0))) %>%
pivot_longer(cols = contains("___"),
names_to = c("metric", "source"),
names_sep = '___', values_drop_na = F) %>%
mutate(fmt_success.label = ifelse(fmt_success.label == 'Failed',
'Unresolved Symptoms', 'Resolved Symptoms'))
sstr_cases.rcdi.rel_abund.annotated <-
sstr_cases.rcdi.metrics %>%
filter(metric == 'rel_abund') %>%
filter(Days_Since_FMT.post <= 37) %>%
# keep only recipient and donor-derived (excluding coexistence)
mutate(
source.simple = case_when(
source %in% c('Recipient / Initial Sample','Self Sp.') ~ 'Recipient',
source %in% c('Donor','Donor Sp.') ~ 'Donor',
T ~ 'Drop'
)) %>%
filter(source.simple != 'Drop') %>%
group_by(Case_Name, Days_Since_FMT.post, source.simple, fmt_success.label) %>%
summarize(value = sum(value, na.rm = T)) %>%
ungroup()
Scatterplot over time
Fraction of donor and recipient-derived species over time for failed and resolved cases
plot <-
sstr_cases.rcdi.rel_abund.annotated %>%
ggplot(aes(Days_Since_FMT.post, value)) +
geom_point(aes(fill = source.simple), alpha = 0.5,
shape = 21, size = 3, col = 'black') +
geom_line(aes(col = source.simple,
group = paste0(Case_Name, source.simple)
), show.legend = F, alpha = 0.5) +
geom_smooth(se = F, method = 'glm', aes(col = source.simple),
method.args=list(family='binomial')) +
geom_hline(yintercept = 0.25, linetype = 'dashed', size = .5) +
scale_color_manual(values = colors.discrete[c(1,3)]) +
scale_fill_manual(values = colors.discrete[c(1,3)]) +
scale_y_continuous(labels = percent_format_signif,
breaks = c(0, .25, .5, .75, 1),
limits = c(0, 1), expand = c(0,0)) +
scale_x_continuous(trans = pseudo_log_trans(0.5, 10),
breaks = c(2, 7, 14, 35, 84, 365)) +
facet_grid(cols = vars(fmt_success.label)) +
theme_cowplot() +
theme(axis.title.x = element_blank(),
axis.line = element_line(size = 0.2, color = 'black'),
strip.text.x = element_text(size = 14),
strip.background = element_blank()) +
panel_border(remove = F, size = 1, color = 'black') +
labs(y = 'Post-FMT Sample\nRel. Abund. by Source') +
guides(fill = guide_legend(title = 'Post-FMT\nMicrobiota Source', ncol = 1))
plot + theme(legend.position = 'none')
legend = cowplot::get_legend(plot)
grid.newpage()
grid.draw(legend)

Exporting plot
output_name = 'PostSource.Cases.Time.rCDI'
ggsave(plot + theme(legend.position = 'none'),
device = 'pdf', dpi = 300, width = 6, height = 3,
filename = paste0(results_dir, fig, output_name, '.Plot.pdf'))
ggsave(legend,
device = 'pdf', dpi = 300, width = 3, height = 3,
filename = paste0(results_dir, fig, output_name, '.Legend.pdf'))
Persistence / Engraftment within time periods:
sstr_cases.rcdi.rel_abund.annotated %>%
filter(Days_Since_FMT.post > 0, Days_Since_FMT.post <= 7) %>%
group_by(source.simple, fmt_success.label) %>%
summarize(mean = mean(value) * 100,
sd = sd(value) * 100,
.groups = 'drop')
sstr_cases.rcdi.rel_abund.annotated %>%
filter(Days_Since_FMT.post > 7, Days_Since_FMT.post <= 37) %>%
group_by(source.simple, fmt_success.label) %>%
summarize(mean = mean(value) * 100,
sd = sd(value) * 100,
.groups = 'drop')
Resolved:
Donor -> 44.8 ± 30.1 to 52.0 ± 23.6
Recipient -> 20.9 ± 24.9 to 12.5 ± 17.0
Failed:
Donor -> 33.1 ± 23.5 to 24.3 ± 8.7
Recipient -> 25.2 ± 19.0 to 40.1 ± 24.3
Boxplot
Boxplots at <=1W, 1-6W, >6W
sstr_cases.rcdi.metrics %>%
filter(metric == 'rel_abund') %>%
mutate(Days_Since_fmt_success.label = case_when(
Days_Since_FMT.post <7 ~ '<1W',
Days_Since_FMT.post >=7 & Days_Since_FMT.post <= 42 ~ '1-6W',
Days_Since_FMT.post > 42 ~ '>6W')) %>%
# keep only recipient and donor-derived (excluding coexistence)
mutate(
source.simple = case_when(
source %in% c('Recipient / Initial Sample','Self Sp.') ~ 'Recipient',
source %in% c('Donor','Donor Sp.') ~ 'Donor',
T ~ 'Drop'
)) %>%
filter(source.simple != 'Drop') %>%
group_by(Case_Name, Days_Since_FMT.post, Days_Since_fmt_success.label, source.simple, fmt_success.label) %>%
summarize(value = sum(value, na.rm = T)) %>%
ungroup() %>%
mutate(Days_Since_fmt_success.label = fct_relevel(Days_Since_fmt_success.label, '<1W', '1-6W', '>6W')) %>%
ggplot(aes(Days_Since_fmt_success.label, value, fill = source.simple)) +
geom_boxplot() +
scale_y_continuous(labels = percent_format_signif) +
facet_grid(cols = vars(gsub(fmt_success.label, pattern = ' ', replacement = '\n')),
space = 'free', scales = 'free_x') +
theme_cowplot() +
theme(axis.title.x = element_blank(),
strip.background = element_blank()) +
scale_fill_manual(values = colors.discrete[c(1,3)]) +
labs(y = 'Cumulative\nSample Rel. Abund.') +
guides(fill = guide_legend(title = 'Post-FMT\nMicrobiota Source', ncol = 2))
Diversity Differences
Alpha: Shannon
Format
# transpose
mp_species.wide <- mp_species.long %>%
filter(kingdom == 'Bacteria',
Study_Type == 'rCDI') %>%
pivot_wider(id_cols = 'species',
names_from = 'Name',
values_from = 'rel_abund',
values_fill = list(rel_abund = 0))
# alpha diversity
mp_species.samples <- t(dplyr::select(mp_species.wide, matches('.pair'), species) %>% column_to_rownames(var = 'species'))
mp_species.shannon <- data.frame(Shannon = diversity(mp_species.samples, index = 'shannon')) %>% rownames_to_column('Name') %>%
left_join(., samples.metadata, by = 'Name')
Boxplots
Shannon Index of rCDI and Control data showing that cases in which FMT failed to resolve clinical symptoms can not be distinguished by controls, successfully treated patients or donors in terms of Shannon Index. Index is significantly lower in pre-FMT samples.
Since MGH03D-Donor (only donor with failed cases) on higher end of Shannon Index, alpha diversity probably not a very relevant indicator for choosing donors.
ww = 0.5
plot <-
mp_species.shannon %>%
filter(!is.na(Sample_Type)) %>%
# filter(last_sample) %>%
mutate(Sample_Type.label = str_to_upper(unlist(str_extract(Sample_Type, '.')))) %>%
mutate(tag = case_when(
Donor.Subject != 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' ~ Sample_Type.label,
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' &
(Sample_Type == 'donor' | (Sample_Type == 'post' & !fmt_success)) ~ paste0(Sample_Type.label, '*'),
T ~ Sample_Type.label
)) %>%
mutate(tag = fct_relevel(tag, 'R', 'P', 'D', 'P*', 'D*')) %>%
# filter(tag != 'R') %>%
ggplot(aes(tag, Shannon)) +
stat_boxplot(geom = 'errorbar', width = ww) +
geom_boxplot(show.legend = F, width = ww) +
geom_dotplot(aes(fill = NULL), binaxis = "y",
stackdir = "center", binpositions="all", binwidth = 0.05) +
theme_cowplot() +
theme(axis.title.x = element_blank()) +
labs(y = 'Shannon Index') +
annotate('text', label = '*Unresolved\nSymptoms', y = 0.45, x = 'D*', hjust = 1) # +
# stat_compare_means(method = 'kruskal')
plot
Exporting plot
output_name = 'TaxonomicDiversity.Shannon'
ggsave(plot + theme(legend.position = 'none'),
device = 'pdf', dpi = 300, width = 2, height = 2.5,
filename = paste0(results_dir, fig, output_name, '.Plot.pdf'))
Beta: PCA of species-level taxonomic composition
PCA of clr-transformed taxonomic composition
Diversity Calculations
# transpose
mp_species.rcdi.wide <- mp_species.rcdi.long %>%
filter(kingdom == 'Bacteria') %>%
pivot_wider(id_cols = 'species',
names_from = 'Name',
values_from = 'rel_abund',
values_fill = list(rel_abund = 0))
## -- genus-level
mp_genus.rcdi.wide <- mp_species.rcdi.long %>%
filter(kingdom == 'Bacteria') %>%
pivot_wider(id_cols = 'genus',
names_from = 'Name',
values_from = 'rel_abund',
values_fill = list(rel_abund = 0),
values_fn = list(rel_abund = sum))
# get data
mp_species.rcdi.data <- t(dplyr::select(mp_species.rcdi.wide,
matches('.pair'), species) %>%
column_to_rownames(var = 'species'))
## -- genus
mp_genus.rcdi.data <- t(dplyr::select(mp_genus.rcdi.wide,
matches('.pair'), genus) %>%
column_to_rownames(var = 'genus'))
# species-level
pseudo = 1e-6
mp_species.rcdi.clr <- rgr::clr(mp_species.rcdi.data + pseudo, ifclose = FALSE, ifwarn = TRUE)
# aitchison dist
mp_species.rcdi.clr.dist <- dist(scale(mp_species.rcdi.clr, center = T, scale = F),
method = 'euclidean') %>% as.matrix()
# pca
mp_species.rcdi.clr.pca_data <- PCA(mp_species.rcdi.clr, graph = FALSE, scale.unit = F)
mp_species.rcdi.clr.pca <- mp_species.rcdi.clr.pca_data %>%
.$ind %>% .$coord %>%
as.data.frame() %>%
rownames_to_column(var = 'Name')
## annotate with metadata, diversity
mp_species.rcdi.data.clr.pca <- mp_species.rcdi.clr.pca %>%
dplyr::left_join(., samples.metadata, by = c('Name')) %>%
dplyr::left_join(., mp_species.shannon %>% dplyr::select(Name, Shannon), by = c('Name')) %>%
dplyr::group_by(Case_Name, Sample_Type) %>%
dplyr::mutate(last = dense_rank(Days_Since_FMT) == max(dense_rank(Days_Since_FMT))) %>%
dplyr::mutate(d3.tag = case_when(
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'post' ~
paste0('post | D3 ', if_else(fmt_success, 'Success', 'Failed')),
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'donor' ~ 'donor | D3',
T ~ Sample_Type)) %>%
dplyr::ungroup()
D/P Difference
PERMANOVA (adonis function) testing between rCDI Donors & Post-FMT: MGH03D with failed cases (3 samples) and other donors.
# donor & post-FMT
selection <- mp_species.rcdi.long %>%
dplyr::filter(Sample_Type %in% c('post', 'donor'), last_sample) %>%
dplyr::distinct(Name, Donor.Subject, Sample_Type, fmt_success)
d3.adonis <-
pairwiseAdonis::pairwise.adonis(
x = mp_species.rcdi.clr[selection$Name, ],
factors = ifelse(selection$Donor.Subject == "D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21", 'MGH03D-related','Other'),
sim.method = 'euclidean',
perm = 1e3) %>%
rename(fdr = p.adjusted) %>%
dplyr::mutate(sig = cut(fdr,
breaks = c(-Inf, 0.001, 0.01, 0.05, Inf),
labels = c("***", "**", "*", "ns")))
d3.adonis
PCA biplot
Format
Abbr. species names in PCA object
select.prevotella <- c('Prevotella_denticola', 'Prevotella_oris', 'Prevotella_oralis','Prevotella_veroralis')
select.fusobacteria <- c('Fusobacterium_nucleatum', 'Fusobacterium_periodonticum', 'Fusobacterium_mortiferum')
other <- c('Rothia_dentocariosa', 'Veilonella_parvula')
select.bacteroides <- c('Bacteroides_faecis', 'Bacteroides_eggerthii', 'Bacteroides_intestinalis', 'Bacteroides_thetaiotaomicron')
# 'Bacteroides_fragilis',
select.ruminococcus <- c('Ruminococcus_torques', 'Ruminococcus_gnavus')
# 'Ruminococcus_albus', 'Ruminococcus_callidus', 'Bacteroides_obeum'
plot_spp <- c(other, select.bacteroides, select.ruminococcus, select.prevotella, select.fusobacteria,
'Lachnospiraceae_bacterium')
print_taxonomy <- function(name, selection) {
unlist(
lapply(name, function(x) {
case_when(
!x %in% selection ~ x,
x %in% selection ~ str_replace(str_replace(x, '_', ' '), '[a-z].*\ ', '. ')
)
}
)) %>%
return()
}
rownames(mp_species.rcdi.clr.pca_data$var$coord) <- print_taxonomy(rownames(mp_species.rcdi.clr.pca_data$var$coord), plot_spp)
rownames(mp_species.rcdi.clr.pca_data$var$cor) <- print_taxonomy(rownames(mp_species.rcdi.clr.pca_data$var$cor), plot_spp)
rownames(mp_species.rcdi.clr.pca_data$var$cos) <- print_taxonomy(rownames(mp_species.rcdi.clr.pca_data$var$cos), plot_spp)
rownames(mp_species.rcdi.clr.pca_data$var$contrib) <- print_taxonomy(rownames(mp_species.rcdi.clr.pca_data$var$contrib), plot_spp)
# https://stackoverflow.com/questions/13407236/remove-a-layer-from-a-ggplot2-chart
remove_geom <- function(ggplot2_object, geom_type) {
# Delete layers that match the requested type.
layers <- lapply(ggplot2_object$layers, function(x) {
if (class(x$geom)[1] == geom_type) {
NULL
} else {
x
}
})
# Delete the unwanted layers.
layers <- layers[!sapply(layers, is.null)]
ggplot2_object$layers <- layers
ggplot2_object
}
pp <-
factoextra::fviz_pca_biplot(mp_species.rcdi.clr.pca_data,
# samples
fill.ind = mp_species.rcdi.data.clr.pca$d3.tag,
pointshape = 21,
pointsize = 8,
# alpha.ind = ifelse(grepl(mp_species.rcdi.data.clr.pca$d3.tag, pattern = 'D3'), 1, .25),
# features
select.var = list('name' = print_taxonomy(plot_spp, plot_spp)),
label = 'var', labelsize = 3,
col.var = 'black',
repel = T, invisible = c("quali"),
# general
addEllipses = F, centroids = F, #scale = F,
title = '',
subtitle = 'Taxonomic Composition') +
ggpubr::fill_palette(palette = c(colors.discrete[c(1,1,2)],
'#B4907E', #A47963
colors.discrete[c(2,3)])) +
scale_x_reverse() +
scale_y_reverse() +
theme_cowplot() +
theme(aspect.ratio = 1,
plot.subtitle = element_text(hjust = .5, size = 12),
axis.title = element_text(size = 10),
axis.text = element_text(size = 8)) +
coord_flip()
# axes - rename
pp$labels$x <- str_replace(pp$labels$x, pattern = 'Dim', replacement = 'PC ')
pp$labels$y <- str_replace(pp$labels$y, pattern = 'Dim', replacement = 'PC ')
# arrows - rescale
pp[["layers"]][[5]][["data"]][["x"]] = pp[["layers"]][[5]][["data"]][["x"]] *.4
pp[["layers"]][[5]][["data"]][["y"]] = pp[["layers"]][[5]][["data"]][["y"]] *.4
# labels
pp[["layers"]][[4]][["data"]][["x"]] = pp[["layers"]][[4]][["data"]][["x"]] *.4
pp[["layers"]][[4]][["data"]][["y"]] = pp[["layers"]][[4]][["data"]][["y"]] *.4
# crosshair
pp <- remove_geom(pp, 'GeomHline')
pp <- remove_geom(pp, 'GeomVline')
pp <- pp +
annotate('text', x = -52, y = 52, hjust = 0, vjust = 1, size = 3,
label = paste0('PERMANOVA (Post-FMT & Donors)\n',
'MGH03D-related vs. Others\n')) +
annotate('text', x = -41.5, y = 52, hjust = 0, vjust = 1, size = 3,
label = bquote(R^2 * '=' * .(format(100*d3.adonis$R2,digits=3))*'%' * .(format(d3.adonis$sig))))
legend <- cowplot::get_legend(pp)
pp + theme(legend.position = 'none')
grid.newpage()
grid.draw(legend)

Exporting plot
output_name = 'TaxonomicDiversity.rCDI.CLR_PCAbiplot'
ggsave(pp + theme(legend.position = 'none'),
device = 'pdf', dpi = 300, width = 5, height = 5,
filename = paste0(results_dir, fig, output_name, '.Plot.pdf'))
ggsave(legend,
device = 'pdf', dpi = 300, width = 5, height = 5,
filename = paste0(results_dir, fig, output_name, '.Legend.pdf'))
Donor Difference
# donor only
donors <- mp_species.rcdi.long %>%
filter(Sample_Type %in% c('donor')) %>%
distinct(Name, Donor.Subject)
selection <- donors %>%
pull(Name)
mp_species.rcdi.clr.pca_data <- PCA(mp_species.rcdi.clr[selection, ], graph = FALSE, scale.unit = F)
# get data
mp_species.rcdi.data <- t(dplyr::select(mp_species.rcdi.wide,
matches('.pair'), species) %>%
column_to_rownames(var = 'species'))
# species-level
pseudo = 1e-6
mp_species.rcdi.donors.data <- mp_species.rcdi.data[selection, colSums(mp_species.rcdi.data[selection,]) > 0]
mp_species.rcdi.donors.clr <- rgr::clr(mp_species.rcdi.donors.data + pseudo, ifclose = FALSE, ifwarn = TRUE)
# PCA
mp_species.rcdi.donors.clr.pca_data <- PCA(mp_species.rcdi.donors.clr, graph = FALSE, scale.unit = F, ncp = 2)
mp_species.rcdi.donors.clr.pca <- mp_species.rcdi.donors.clr.pca_data %>%
.$ind %>% .$coord %>%
as.data.frame()
mp_species.rcdi.donors.clr.expl <- mp_species.rcdi.donors.clr.pca_data$eig[1:2,'percentage of variance'] / 100
## annotate with metadata, diversity
mp_species.rcdi.donors.data.clr.pca <- mp_species.rcdi.donors.clr.pca %>%
rownames_to_column('Name') %>%
left_join(., samples.metadata, by = c('Name')) %>%
left_join(., mp_species.shannon %>% dplyr::select(Name, Shannon), by = c('Name')) %>%
group_by(Case_Name, Sample_Type) %>%
mutate(last = dense_rank(Days_Since_FMT) == max(dense_rank(Days_Since_FMT))) %>%
mutate(d3.tag = case_when(
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'post' ~
paste0('post | D3 ', if_else(fmt_success, 'Success', 'Failed')),
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'donor' ~ 'donor | D3',
T ~ Sample_Type)) %>%
ungroup()
Pairwise PERMANOVA (adonis function) testing between rCDI Donors: MGH03D with failed cases (3 samples) and other donors.
d3.adonis <-
pairwiseAdonis::pairwise.adonis(
x = mp_species.rcdi.clr[donors$Name, ],
factors = ifelse(donors$Donor.Subject == "D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21", 'MGH03D','Other'),
sim.method = 'euclidean',
p.adjust.m = 'fdr',
perm = 1e3) %>%
mutate(R = sqrt(R2)) %>%
rename(p.bonferroni = p.adjusted)
d3.adonis
# aitchison dist
mp_species.rcdi.donors.clr.dist <- dist(scale(mp_species.rcdi.donors.clr, center = T, scale = F),
method = 'euclidean') %>% as.matrix()
mp_species.rcdi.donors.clr.dist.long <- mp_species.rcdi.donors.clr.dist %>%
as.data.frame(.) %>%
distmat_to_long(., value_name = 'dist', rm_diag = T) %>%
left_join(., samples.metadata %>% dplyr::select(Name, Donor.Unique_ID, Donor.Subject),
by = c('row' = 'Name')) %>%
left_join(., samples.metadata %>% dplyr::select(Name, Donor.Unique_ID, Donor.Subject),
by = c('col' = 'Name'), suffix = c('.row','.col')) %>%
mutate(d3.tag = Donor.Subject.row == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' |
Donor.Subject.col == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21'
) %>%
filter(Donor.Subject.row != Donor.Subject.col)
mp_species.rcdi.donors.clr.dist.long %>%
mutate(d3.tag = ifelse(d3.tag, 'MGH03D\nOther','Other\nOther')) %>%
ggplot(aes(x = (dist - mean(mp_species.rcdi.donors.clr.dist.long$dist)) /
sd(mp_species.rcdi.donors.clr.dist.long$dist),
fill = d3.tag)) +
geom_density(alpha = 0.5) +
scale_y_continuous(breaks = c(0, .25, .5),
labels = percent_format()) +
geom_vline(xintercept = 1, linetype = 'dashed', alpha = 0.5) +
theme_cowplot() +
theme(aspect.ratio = 1,
legend.position = 'top',
legend.title = element_blank()) +
labs(x = 'Beta-Diversity (Z-Score)')
output_name = 'TaxonomicDiversity.CLR_Euc.MGH03D.'
ggsave(plot + theme(legend.position = 'none'),
device = 'pdf', dpi = 300, width = 2.5, height = 4,
filename = paste0(results_dir, fig, output_name, '.Plot.pdf'))
Taxa Distinguishing MGH03D-related / Other
Format
mp_species.rcdi.annotated <-
samples.metadata %>%
mutate(tag = paste0(Unique_ID, ' ', gsub(
gsub(Name, pattern = '.*_', replacement = ''),
pattern = '.pair', replacement = ''))) %>%
right_join(., as.data.frame(mp_species.rcdi.data) %>% rownames_to_column('Name')) %>%
filter(Sample_Type %in% c('post','donor'))
X.metadata <-
mp_species.rcdi.annotated %>%
dplyr::select(Study:tag) %>%
mutate(Y = ifelse(Donor.Subject == "D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21", 'MGH03D-related', 'Other'))
X =
mp_species.rcdi.annotated %>%
column_to_rownames(var = 'tag') %>%
dplyr::select(-Study:-Study_Type) %>%
as.data.frame()
Y = as.factor(X.metadata$Y)
sparse PLS Discriminant Analysis
Initial sPLS-DA
sPLS-DA of rCDI recipients and donor samples based on taxonomy
pseudo = 1e-6
data.plsda = mixOmics::plsda(X = X + pseudo, Y, ncomp = nlevels(Y), logratio = 'CLR')
data.perf.plsda = mixOmics::perf(data.plsda, validation = 'Mfold', folds = 5,
progressBar = FALSE, nrepeat = 10)
plot(data.perf.plsda, overlay = 'measure', sd=TRUE)
mixOmics::plotIndiv(data.plsda , comp = c(1,2),
group = Y,
ind.names = T,
ellipse = T,
legend = TRUE, title = 'PLSDA comp 1 - 2')
Cross-Validate features
5x10 CV
seed = 100
set.seed(seed)
data.tune.splsda = mixOmics::tune.splsda(X + pseudo,
Y = Y,
ncomp = 2,
multilevel = NULL,
logratio = 'CLR',
validation = c('Mfold'),
folds = 5,
dist = 'max.dist',
nrepeat = 10,
progressBar = FALSE)
plot(data.tune.splsda)
select.keepX = data.tune.splsda$choice.keepX[1:2]
select.keepX
Apply CV features
data.splsda = mixOmics::splsda(X = X + pseudo, Y = Y,
ncomp = 2,
keepX = select.keepX,
logratio= "CLR",
near.zero.var = T,
)
data.perf.splsda = mixOmics::perf(data.splsda, validation = 'Mfold', folds = 5,
progressBar = FALSE, nrepeat = 10, dist = 'max.dist')
data.perf.splsda$error.rate
mixOmics::plotIndiv(data.splsda , comp = c(1,2),
group = Y, ind.names = T,
ellipse = TRUE, legend = TRUE, title = 'PLSDA comp 1 - 2')
Plot loadings and save to table
# COMP 1
pL.pc1 <- mixOmics::plotLoadings(data.splsda, title = 'sPLS-DA PC 1',
comp = 1, method = 'median', contrib = 'max',
size.title = rel(1), border = T,
size.name = .5, size.legend = .75,
legend.color = colors.discrete[c(4, 1)])
pL.pc1
# COMP 2
pL.pc2 <- mixOmics::plotLoadings(data.splsda, title = 'sPLS-DA PC 2',
comp = 2, method = 'median', contrib = 'max',
size.title = rel(1), border = T,
size.name = .5, size.legend = .75,
legend.color = colors.discrete[c(4, 1)])
pL.pc2
pL <-
rbind(pL.pc1$X, pL.pc2$X) %>%
rownames_to_column('species') %>%
left_join(., microbe.metadata, by = 'species') %>%
rename(rel_abund.highest = GroupContrib) %>%
dplyr::select(species,
habit.site,
oxygen.class,
MGH03D.related,
Other,
rel_abund.highest,
importance)
output_name = 'sPLSDA.MGH03D.Taxonomic.Loadings'
write_tsv(pL, paste0(results_dir, fig, output_name, '.tsv'))
Heatmap plot of differentially abundant taxa
MGH03D-related:
- Prevotella and Bacteroides/Parabacteroides-related species
- Odoribacter, Megasphaera, Alistipes, Desulfovibrio, Bacteroidales, Fusobacterium (mortiferum), Megamonas
- R. torques, Lachnospiraceae, some Eubacterium species
- Lachnospiraceae, Eggerthella, Anaerostipes, Streptococci
This is supported by strain-level transfer showing that in MGH03D-related cases Prevotella, Parabacteroides, Megasphaera, Alistipes and Odoribacter are transferred
Link to sulfate metabolism? IBD?
sample_colors <-
tibble(tag = data.splsda$names$sample) %>%
left_join(., X.metadata) %>%
mutate(color =
case_when(Y == 'MGH03D-related' & Sample_Type == 'donor' ~ colors.discrete[1],
Y != 'MGH03D-related' & Sample_Type == 'donor' ~ colors.discrete[6],
Y == 'MGH03D-related' & Sample_Type == 'post' & fmt_success ~ colors.discrete[2],
Y == 'MGH03D-related' & Sample_Type == 'post' & !fmt_success ~ '#B4907E',
Y != 'MGH03D-related' ~ colors.discrete[7],
T ~ 'white'))
output_name = 'sPLSDA.MGH03D.Taxonomic.Heatmap'
mixOmics::cim(data.splsda, row.sideColors = sample_colors$color,
symkey = T, keysize = c(0.8, 0.8),
row.names = F,
col.names = gsub(data.splsda$names$colnames$X, pattern = '_noname', replacement = ''),
save = 'pdf',
name.save = paste0(results_dir, fig, output_name),
margins = c(25, 10),
row.cex = .5,
transpose = T,
scale = T, center = T)
Functions Distinguishing MGH03D-related / Other
Format
h2_unstratified <- samples.metadata %>%
right_join(., read_tsv(paste0(data_dir, 'samples.h2_profiles.unstratified.tsv')),
by = 'Name')
Annotate Humann2 with PWY Classes
h2.rcdi.wide <-
h2_unstratified %>%
filter(!is.na(pathway_description)) %>%
reshape2::dcast(., pathway_id + pathway_description ~ Name,
value.var = 'CPM', fun.aggregate = sum, na.rm = T)
# transpose
h2.rcdi.wide <- h2_unstratified %>%
filter(!is.na(pathway_description)) %>%
pivot_wider(id_cols = 'pathway_description',
names_from = 'Name',
values_from = 'CPM',
values_fill = list(CPM = 0))
# get data
h2.rcdi.data <- t(dplyr::select(h2.rcdi.wide,
matches('.pair'), pathway_description) %>%
column_to_rownames(var = 'pathway_description'))
Diversity
Diversity metrics based on species-level relative abundances
Alpha: Shannon Index
Alpha Diversity
h2.rcdi.shannon <- data.frame(Shannon = diversity(h2.rcdi.data, index = 'shannon')) %>% rownames_to_column('Name')
CLR-Transform
pseudo = 1e-3 # CPM
h2.rcdi.clr <- rgr::clr(h2.rcdi.data + pseudo, ifclose = FALSE, ifwarn = TRUE)
Beta: PCA of functional composition
h2.rcdi.clr.pca_data <- PCA(h2.rcdi.clr, graph = FALSE, scale.unit = F)
h2.rcdi.clr.pca <- h2.rcdi.clr.pca_data %>%
.$ind %>% .$coord %>%
as.data.frame() %>%
rownames_to_column(var = 'Name')
## annotate with metadata, diversity
h2.rcdi.data.clr.pca <- h2.rcdi.clr.pca %>%
left_join(., samples.metadata, by = 'Name') %>%
left_join(., h2.rcdi.shannon, by = 'Name') %>%
group_by(Case_Name, Sample_Type) %>%
mutate(last = dense_rank(Days_Since_FMT) == max(dense_rank(Days_Since_FMT))) %>%
mutate(d3.tag = case_when(
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'post' ~
paste0('post | D3 ', if_else(fmt_success, 'Success', 'Failed')),
Donor.Subject == 'D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21' & Sample_Type == 'donor' ~ 'donor | D3',
T ~ Sample_Type)) %>%
ungroup()
plot <-
h2.rcdi.data.clr.pca %>%
ggplot(aes(Dim.2, Dim.1, label = Unique_ID)) +
geom_point(shape = 21, col = 'black', size = 8,
aes(fill = Sample_Type)) + # Sample_Type
scale_fill_manual(values = c(colors.discrete[c(1,2,3)]),
guide = guide_legend(reverse = TRUE)) +
theme_cowplot() +
labs(subtitle = 'Functional Composition') +
coord_fixed() +
theme(plot.subtitle = element_text(hjust = 0.5),
legend.title=element_blank(),
aspect.ratio = 1
) +
labs(y = paste0('PC 1 (', round(h2.rcdi.clr.pca_data$eig[1,'percentage of variance'], 1), '%)'),
x = paste0('PC 2 (', round(h2.rcdi.clr.pca_data$eig[2,'percentage of variance'], 1), '%)'))
legend <- cowplot::get_legend(plot)
plot + theme(legend.position = 'none')
grid.newpage()
grid.draw(legend)
Format
h2.rcdi.annotated <-
samples.metadata %>%
mutate(tag = paste0(Unique_ID, ' ', gsub(
gsub(Name, pattern = '.*_', replacement = ''),
pattern = '.pair', replacement = ''))) %>%
right_join(., as.data.frame(h2.rcdi.data) %>% rownames_to_column('Name')) %>%
filter(Sample_Type %in% c('post','donor')) %>%
mutate(Y = ifelse(Donor.Subject == "D0_ALM_Case_4;5;6;8;9;12;13;15;17;18;20;21", 'MGH03D-related', 'Other'))
X.metadata <-
h2.rcdi.annotated %>%
dplyr::select(Study:tag, Y)
X =
h2.rcdi.annotated %>%
column_to_rownames(var = 'tag') %>%
dplyr::select(-Study:-Study_Type, -Y) %>%
as.data.frame()
Y = as.factor(X.metadata$Y)
sparse PLS Discriminant Analysis
Initial sPLS-DA
sPLS-DA of rCDI recipients and donor samples based on taxonomy
pseudo = 1e-3
data.plsda = mixOmics::plsda(X = X + pseudo, Y, ncomp = nlevels(Y), logratio = 'CLR')
data.perf.plsda = mixOmics::perf(data.plsda, validation = 'Mfold', folds = 5,
progressBar = FALSE, nrepeat = 10)
plot(data.perf.plsda, overlay = 'measure', sd=TRUE)
mixOmics::plotIndiv(data.plsda , comp = c(1,2),
group = Y,
ind.names = T,
ellipse = T,
legend = TRUE, title = 'PLSDA comp 1 - 2')
Cross-Validate features
5x10 CV
set.seed(seed)
data.tune.splsda = mixOmics::tune.splsda(X + pseudo,
Y = Y,
ncomp = 2,
multilevel = NULL,
logratio = 'CLR',
validation = c('Mfold'),
folds = 5,
dist = 'max.dist',
nrepeat = 10,
progressBar = FALSE)
plot(data.tune.splsda)
select.keepX = data.tune.splsda$choice.keepX[1:2]
select.keepX
# select.keepX = c(20, 20)
Apply CV features
data.splsda = mixOmics::splsda(X = X + pseudo, Y = Y,
ncomp = 2,
keepX = select.keepX,
logratio= "CLR",
near.zero.var = T,
)
data.perf.splsda = mixOmics::perf(data.splsda, validation = 'Mfold', folds = 5,
progressBar = FALSE, nrepeat = 10, dist = 'max.dist')
data.perf.splsda$error.rate
mixOmics::plotIndiv(data.splsda , comp = c(1,2),
group = Y, ind.names = T,
ellipse = TRUE, legend = TRUE, title = 'PLSDA comp 1 - 2')
Get colors for sample columns and taxa rows
sample_colors <-
tibble(Unique_ID.splsda = str_split_fixed(data.splsda$names$sample, pattern = ' ', n = 2)[,1]) %>%
cbind(., h2.rcdi.annotated) %>%
mutate(color =
case_when(Y == 'Other' & Sample_Type == 'post' ~ colors.discrete[7],
Y == 'Other' & Sample_Type == 'donor' ~ colors.discrete[6],
Y == 'MGH03D-related' & Sample_Type == 'post' & fmt_success ~ colors.discrete[2],
Y == 'MGH03D-related' & Sample_Type == 'post' & !fmt_success ~ '#B4907E',
Y == 'MGH03D-related' & Sample_Type == 'donor' ~ colors.discrete[1],
T ~ 'white'))
Plot loadings and save to table
# COMP 1
pL.pc1 <- mixOmics::plotLoadings(data.splsda, title = 'sPLS-DA PC 1',
comp = 1, method = 'median', contrib = 'max',
size.title = rel(1), border = T,
size.name = .5, size.legend = .75,
legend.color = colors.discrete[c(4, 1)])
pL.pc1
# COMP 2
pL.pc2 <- mixOmics::plotLoadings(data.splsda, title = 'sPLS-DA PC 2',
comp = 2, method = 'median', contrib = 'max',
size.title = rel(1), border = T, margins = c(0, 0),
size.name = .5, size.legend = .75,
legend.color = colors.discrete[c(4, 1)])
pL.pc2
pL <-
rbind(pL.pc1$X, pL.pc2$X) %>%
rownames_to_column('pathways') %>%
rename(rel_abund.highest = GroupContrib) %>%
dplyr::select(pathways,
MGH03D.related,
Other,
rel_abund.highest,
importance)
output_name = 'sPLSDA.MGH03D.Functional.Loadings'
write_tsv(pL, paste0(results_dir, fig, output_name, '.tsv'))
output_name = 'sPLSDA.MGH03D.Functional.Heatmap'
mixOmics::cim(data.splsda,
symkey = T, keysize = c(0.8, 0.8),
row.names = F,
row.sideColors = sample_colors$color,
col.names = gsub(data.splsda$names$colnames$X, pattern = '_noname', replacement = ''),
save = 'pdf',
name.save = paste0(results_dir, fig, output_name),
margins = c(33, 15), row.cex = .35, col.cex = .35,
# margins = c(25,20), row.cex = .8, col.cex = .8,
transpose = T,
# row.cex = .5, col.cex = .75,
# margins = c(6.5, 20),
scale = F, center = T)